Beyond evolution
In this first post of Emergent Properties, Dan and Rahul tackle perhaps the most fundamental question in biology - how do individuals and populations adapt to their environments? They explore the advantages and tradeoffs of evolution via genetic mutation and demonstrate how organisms can develop non-genetic mechanisms to develop adaptation 'shortcuts'. With examples ranging from bacterial nutrient sensing to immune receptor diversity to cancer, they synthesize a framework through which to understand the many different modes of biological adaptation.
We wanted to start Emergent Properties with a post exploring adaptation. Every organism, from single cells to humans, is constantly adapting to the environment, whether it is changes in genetic programs to respond to new nutrients, or using acid-base buffers in blood to maintain pH, or adjusting human behavior based on social norms of a new situation. Evolution and selection provide a core mechanism by which biological systems can change across generations in response to environmental changes. It’s a concept that many of us learn in introductory biology classes - that organisms adapt to the environment around them via mutation1. These mutations, over enough time, can unlock a dizzying array of new biology – animals using sonar for navigation, bacteria capable of metabolizing man-made materials like nylon, fish capable of generating light in the deep sea. Though evolution is powerful, yielding the vast diversity in life we see today, it also has limitations. For instance, it acts on timescales of many generations, and rapid stressors can outpace a population’s ability to adapt (e.g., antibiotics eradicating a population of bacteria). It is a “state-dependent” process, meaning that future evolutionary trajectories are constrained by the current evolutionary “state” of the population. The core mechanism of genetic evolution – random mutation – yields changes that are more often pathological than beneficial. This yields a natural tension – how can organisms develop mechanisms to adapt to a changing environment sufficiently quickly despite these limitations? This led to our post here, where we try to develop an approach for thinking about mechanisms of “adaptability” – the ability of cells and organisms to adjust to changing conditions – through the lens of constraints on genetic evolution.
This post is roughly divided into three parts. The first part describes a framework for how we think about evolution and selection using basic population genetics. The second part focuses on specific examples of adaptation through evolution across and within individuals. Finally, the third part, which (we feel) is our most speculative and compelling, turns to the development of “faster” non-genetic methods of adaptation as a means to address these tradeoffs.
Please note that this is not intended to be a comprehensive survey of evolutionary biology and/or population genetics, but rather meant to speculate on how to conceptually fit genetic evolution into a broader framework of adaptation.
Part 0: A toy example
What if the only way that we could adapt to the environment was through a genetic mutation - specifically, through modifying a DNA base to another DNA base? In such a world, if there is a change in the ambient temperature (which can cause protein denaturation and numerous other problems), the organism would not be able to adjust until replication occurs, mutations are introduced, and an offspring inherits mutation(s) that allow it to function more effectively. There are multiple methods for optimizing such a limited system of adaptability, including having a vast, genetically diverse population (such that the probability of an existing member of the population being better adapted to the new environment is high) and a rapid mutation rate (with a correspondingly rapid replication rate). However, there is a cost to relying on random mutations alone, including the need for a mutation to arise (at random) that makes the population better suited to the new condition. We will discuss this at length in the subsequent sections, but it is telling that some of the most basic organisms have developed other means of dealing with temperature shock. For instance, temperature-responsive transcriptional programs help prevent protein denaturing at various temperatures and prevent the organism and population from relying on genetic changes to adapt to each new condition.
Part 1: A framework for evolution
To set the stage, we will start by defining evolution as we conceptualize it. Evolution, rooted in Darwin’s On the Origin of Species (1859) [1], is closely related to an idea within Ancient Greek philosophy which posited that processes related to chance led to development of the natural world [2]. This development of species through random processes is central to Darwin’s theory of evolution [3]. At its most simple, Darwin’s theory of evolution is the process by which inherited (genetic) traits in a population change over generations. We can think of this as a random walk Markov chain, as each individual carries some probability of (1) acquiring a variant (whether deleterious or advantageous) and (2) passing on that trait to an offspring, with greater weight placed on a variant promoting fitness. We consider each node to be a different phenotype in the population (defined broadly) and the edges represent the transition probabilities.
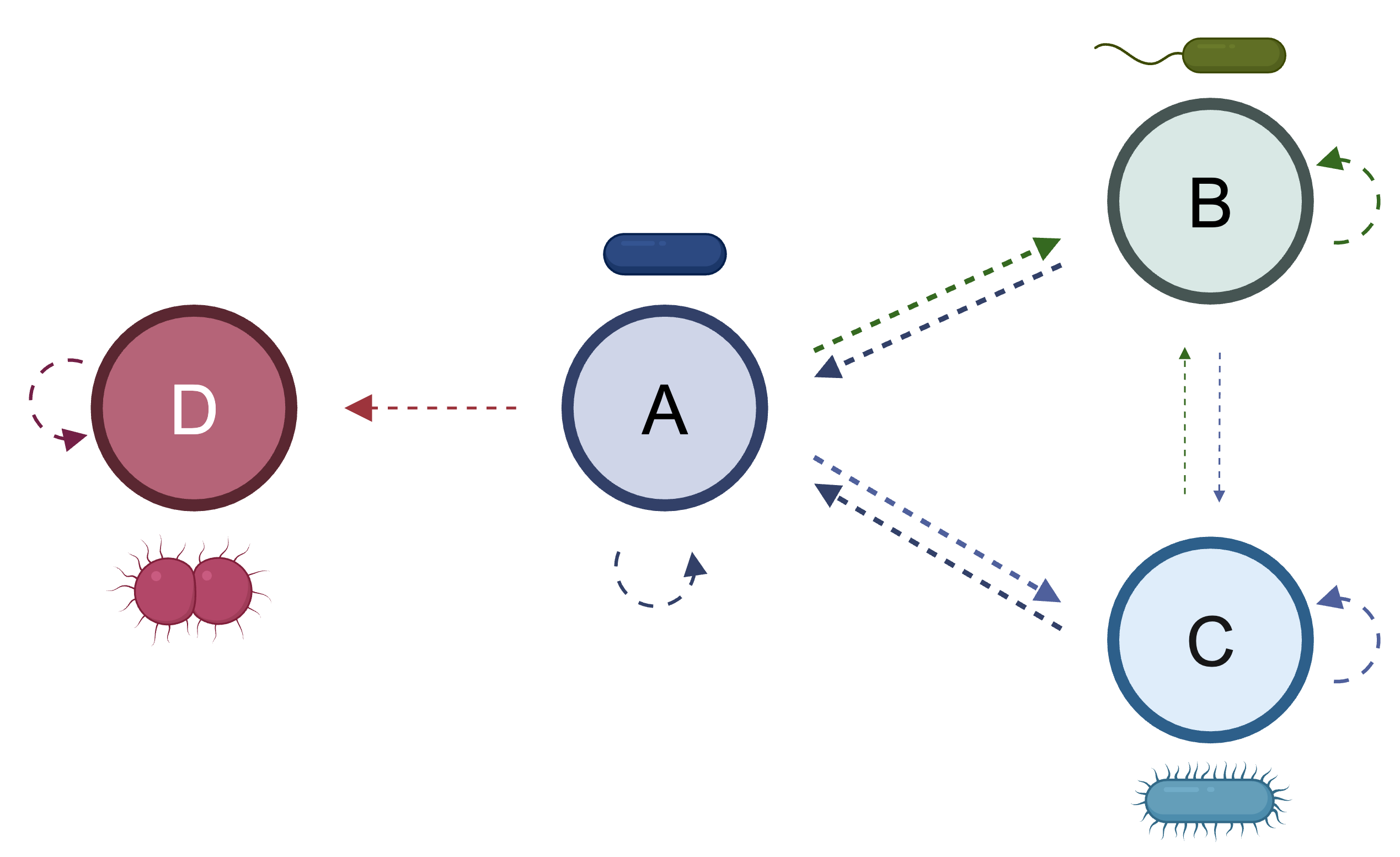 Figure 1 - Evolution as Markov chain. In a Markov chain, a transition can occur between states (for example, between A and B in the figure above) with some probability. Once transitioned to state B, the probability of reverting to state A or transitioning to state C is only dependent on the current state of the system (at B) and is therefore not dependent on having started at A. This figure displays the evolutionary path of a bacterial strain, with each node representing a different phenotype (cell shape/motility) and each edge representing the probability of transitioning to a different phenotype. 2
Figure 1 - Evolution as Markov chain. In a Markov chain, a transition can occur between states (for example, between A and B in the figure above) with some probability. Once transitioned to state B, the probability of reverting to state A or transitioning to state C is only dependent on the current state of the system (at B) and is therefore not dependent on having started at A. This figure displays the evolutionary path of a bacterial strain, with each node representing a different phenotype (cell shape/motility) and each edge representing the probability of transitioning to a different phenotype. 2
From this conceptualization we can see how genetic mutations propagate over generations. New mutations arise randomly as a function of the mutation rate. An existing mutation that promotes fitness will likely become more common with subsequent generations - in other words, fitness advantages occur at the level of phenotypes, impacting the transition probailities in favor of a trait conferring more fitness. An existing mutation can also increase or change in frequency even if it confers no fitness advantage by random chance alone – this process is called random genetic drift. As implied by the binomial distribution, the strength of drift declines as population size increases (a larger population size reduces the probability of major shifts in allele frequencies due to random chance alone). Importantly, these forces act to shift the genetic makeup of populations rather than acting at the level of the individual (we will discuss this more later on). Notably, all of this discussion has been independent of time - as long as mutations occur within some interval, then drift will occur as time stretches to infinity.
The framing of evolution as a Markov chain demonstrates how evolutionary solutions exist in the context of the current state of the population 3. Even in a population where only advantageous mutations develop, some organisms will probabilistically reach a point at which it functionally becomes too expensive to backtrack (even if the chain is ergodic), despite a different state or path being more optimal. More optimal paths have functionally become unreachable, termed an evolutionary “dead end” 4. This is similar to getting stuck in local optima with gradient-based algorithms when searching for the global optimum, and is a significant limitation of both gradient algorithms and evolution itself.
How, then, do we know how fast organisms mutate and if they are undergoing selection? One tool to understanding the rate of evolution is so-called neutral sites 5 – variation in the genome that is thought to not undergo selection. The “neutral theory” of evolution, initially proposed by Kimura in 1968 [5], provides a mathematical framework for understanding the expected behavior of mutations that do not provide a selective advantage or disadvantage 6. Changes in the frequency of such neutral variation, by definition, is via genetic drift and not by selection. By identifying the speed at which new mutations arise in these regions we can estimate mutation rates. If genetic changes in the population over time are different from what we would expect under drift, it may suggest ongoing selective pressures.
Part 2: The promotion of genetic diversity in populations and individuals
An important conclusion from the above basic model of evolution is that the existence of variation is a prerequisite for selection. In a population with a mutation rate of zero, it would be impossible to explore the space of genetic adaptations beyond existing genetic diversity. At the same time, a high mutation rate is likely deleterious as most non-neutral mutations likely have a negative impact on fitness [9]. Thus, there is likely some ideal mutation rate that balances the benefits of genetic diversity at a population scale with the fitness consequences of an increased rate of deleterious mutations.
The presence of a “reservoir” of genetic diversity in a population is important as selection can only act on mutations that exist. In the microbial world, HIV is a very famous example of an elevated mutation rate (>10,000x higher than the human genome) in service of immune and treatment evasion. This mutation rate coupled to a large population size leads to a huge reservoir of genetic diversity where at any given time there is a high chance that a drug-naive population of HIV will have subpopulations resistant to a particular antiviral drug. This results in the need for therapy with two or more classes of drug to prevent the selection of clones which are inherently drug resistant. In the case of HIV, there is clearly pressure in favor of an elevated mutation rate despite the risk of increased deleterious mutations. HIV may come closest to our toy example in Part 0 - an organism that has a very large effective population size and a rapid mutation rate allowing it to use genetic mutation as a primary means to respond to the environment.
A more subtle example of the maintenance of genetic diversity is the HLA locus in humans. This region of chromosome 6 contains several genes which are important for immune cell binding to fragments of proteins; this binding is required for the ability of the immune system to detect if a protein belongs to a foreign pathogen. This region has been shown to be under clear balancing selection, with an increased mutation rate compared to expectation [10]. An increased degree of diversity at this site ensures that if a novel pathogen arises, certain human subpopulations may be better able to recognize the pathogen. Indeed, in the context of SARS-CoV2, certain HLA variants with poorer binding to viral peptides were associated with increased disease risk [11].
What about variation that occurs within a single individual organism? A fascinating example of evolution within an organism is V(D)J recombination in the context of the adaptive immune system. To simplify a complex system, this is how B- and T-cells randomly recombine segments of DNA to produce a huge reservoir of receptors which can detect never-before-seen pathogens. Once a novel pathogen is encountered, an immune cell carrying a “randomly-generated” receptor that best recognizes the pathogen is identified and amplified, allowing for the production of antibodies specific to novel pathogens. Here, the population undergoing evolution and selection is the population of B- and T-cells, however the resultant effect is that the organism (e.g., the human) benefits.
Cancer is another example of within-host evolution which highlights the balance between the fitness of an individual (the cell) and the organism. There is substantial evidence that preventing the development of cancer is one of the major driving forces in organismal evolution. Traditional models for cancer development typically assume a similar path as in figure 1, focusing on a single clone that, through branching evolution, develops sequential tumor suppressor mutations that lead to a replicative advantage over nearby non-precancerous cells while inhibiting growth of nearby healthy cells. Intriguingly, a recent finding using a mouse model of colon cancer (which is often thought to be clonal) suggests that there can be “clonal cooperation” between different pre-cancerous lineages [12], adding complexity to the landscape of selective forces and strengthening the view of cancerous cells as a community of cells with differing functions serving to boost replicative fitness over healthy tissue 7.
We find the above examples interesting because despite their breadth, they can all be understood by the framework of evolution we described in Part 1. Evolutionary processes occurring within our bodies, such as via V(D)J recombination, still follow the same principles of acting at the level of a population (immune cells), but have been harnessed by a single organism (a human) to achieve individual-level benefits. This is one way by which the machinery of evolution, which is limited to act on populations, can be leveraged for the fitness of an individual.
Part 3: Individual adaptation: searching beyond classical evolution
Throughout the above sections we have alluded to several limitations to evolution, the most significant of which is that evolutionary processes typically require multiple generations of organisms and (with some exceptions like V(D)J) act at the level of the population rather than individuals. Rapid stressors may outpace evolution entirely and cause the entire population to be wiped out. A possible way to address this could be to increase the mutation rate, expanding the amount of genetic diversity present in a single generation and theoretically reducing the number of generations required to adapt. So, what happens when we increase mutation rates in complex organisms? In general, damage to enzymes that promote replication fidelity produce severely pathologic consequences. In humans, several rare inherited syndromes of premature aging are caused by impairments in DNA repair. Increased DNA mutagenesis can also damage mechanisms involved in restricting cellular proliferation, increasing the risk for cancers (this is the mechanism by which increased sun exposure increases melanoma risk). In most organisms, the deleterious consequences of random mutagenesis limit the ability of mutagenesis to provide the sole means by which populations can adapt to stressors 8.
Of course, individual organisms have ways to handle stress beyond waiting for random mutations to boost fitness. For example, bacteria such as E. coli evolved the canonical lac operon which is a system by which transcription of genes required to metabolize lactose is only enabled on detection of lactose, preventing wasteful transcription while ensuring that the organism can metabolize this energy source when available. In the context of exposure to heat, bacteria can produce a number of chaperone proteins which help maintain protein folding and function. These are all genetically encoded functions (i.e., that evolved) which operate at timescales much shorter than that of de novo evolution.
Thus, we highlight a distinction between two mechanisms of adaptation: (1) genetic selection, where a response to a stressor requires a shift in the population genetic code resulting in greater fitness, and (2) non-heritable “phenotypic plasticity,” where a single organism can respond to an environmental change using some existing mechanism. If we think about adaptability in the same Markov model as shown in Figure 1, phenotypic plasticity allows for more “rapid” transitions between states, allowing for responses to canonical stressors and preservation of individual organismal integrity and by extension the population. One can imagine that if a population of bacteria is frequently exposed to both lactose and glucose as an energy source, evolution of the lac operon as a shortcut between the lactose-metabolizing and glucose-metabolizing states would be highly optimal.
Another major distinction between genetic evolution and phenotypic plasticity is their relative scope. Genetic evolution explores new nodes in the Markov chain, while phenotypic plasticity is the outcome of evolved “shortcuts” between existing states in the chain. More simply, genetic evolution operates over a much broader space than systems that drive phenotypic plasticity. However, phenotypic plasticity is efficient and fast. To use a corollary from computer science, genetic evolution is similar to writing new code to address a problem, while phenotypic plasticity is like using an existing function or library. Returning to the lac operon, this tool functions in the context of lactose only, allowing efficient metabolism of lactose in a glucose-poor environment. However, this operon cannot function to metabolize arbitrary carbohydrates, for which evolution of a new operon would be required.
V(D)J recombination is a particularly interesting example to view through this framework. At the level of individual immune cells, it is an example of genetic evolution. Across the population of immune cells, a huge space of possible receptors for potentially pathogenic molecules is sampled and fitness is conferred to cells that can best bind the foreign antigen. However, at the level of the whole organism, it is an example of phenotypic plasticity. It is a specialized, pre-evolved system which acts at the level of the individual to switch between “states” that confer an organism immunity to particular pathogens. While it provides shortcuts between a vast number of states (i.e., conferring immunity to virtually arbitrary pathogens), there are states that are outside the scope of the system. For instance, simplistically, certain small molecules produce no immune response, and defense against these molecules would require genetic evolution to develop a novel mechanism for detection and removal.
The most broadly adaptable system which we argue is an example of phenotypic plasticity is the central nervous system. The complexity of the human brain and its ability to learn allows for rapid behavioral adaptation to novel stimuli which act at the level of an individual but are not directly genetically encoded. Similar to other evolved systems conferring phenotypic plasticity, there are theoretical limits – for instance, our ability to sense only visible range light – which have a genetic basis. However, unlike other such systems, the CNS affords us ways around these limitations – for instance we have developed infrared glasses to expand the range of visible light.
The human brain has unique parallels to some core principles of genetic evolution. Aspects of speech / written communication bear some resemblance to basic genetics principles such as the transfer of information and knowledge from person-to-person (like horizontal gene transfer, the transfer of genetic material from organism-to-organism without replication) and from generation-to-generation (like genetic inheritance). Thanks to our ability to transfer the knowledge to build and use such tools to others both horizontally and vertically, the net effect of one person developing a new tool is actually quite similar to genetic evolution. Using the example of visible light, one could imagine pressure to expand the range of visible light in a simpler organism resulting in a selective advantage for individuals with the ability to sense new spectra. After many generations, this would result in the population being able to sense an expanded range of light. Similarly but much more rapidly, the development of infrared glasses and the dissemination of this knowledge to the rest of the population has a similar effect, allowing the population and its descendants to see new light spectra. The human brain is the foremost example of a genetically evolved system which is sufficiently adaptable so as to address nearly arbitrary problems, similar to the role of genetic evolution in most other organismal populations.
The development of the principles of evolution are one of the greatest (and arguably, one of the most recent) major paradigm shifts in biology. Here, we sought to further expand the reach of these principles, using a Markov model-based framework to contextualize all aspects of adaptation, both genetic-and non-genetic. In brainstorming and writing this post, we come away all-the-more compelled by the importance of understanding the paths by which the biological world has come to exist.
References
Acknowledgements
Additional thanks to Kevin Procoipo and Daniel Shur for a helpful critical reading.